Sales partners
Our exclusive partners
BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care.
The company develops innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers.
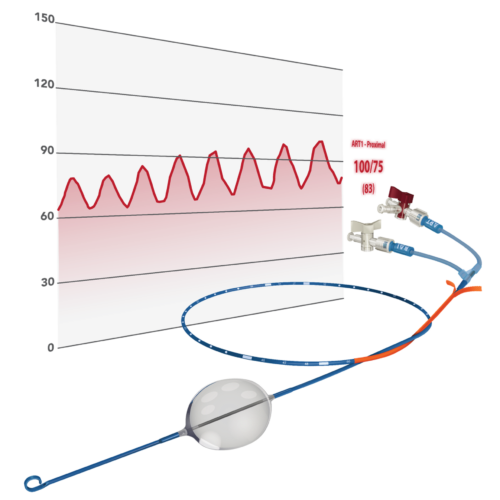
Its TRUE™ Flow Valvuloplasty Perfusion and TRUE™ Dilatation Balloon Valvuloplasty Catheters were designed to provide precise, reliable and controlled dilatations during TAVI and BAV procedures.
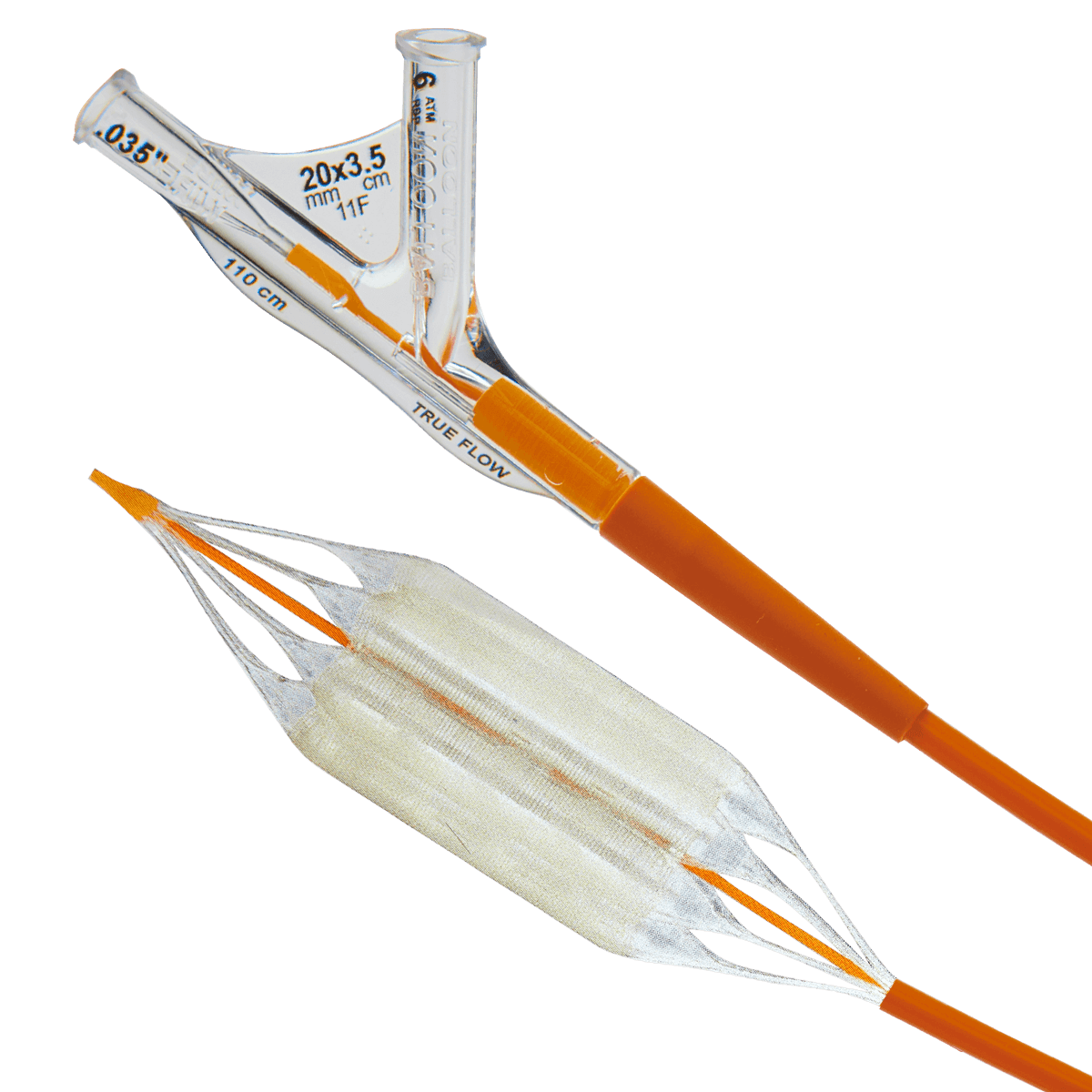
TRUE™ FLOW
The TRUE™ Flow Valvuloplasty Perfusion Catheter uses a unique eight-chamber inflation system to allow continuous cardiac blood flow through the completely inflated balloon.
The balloon was designed to provide low hemodynamic resistance; it is the only balloon providing continuous cardiac blood flow independent of the heart’s rhythmic state.
With its fiber-based shell the TRUE™ Flow balloon is rupture resistant, stays true to size and exhibits less than 1% stretch between nominal and rated burst pressure.
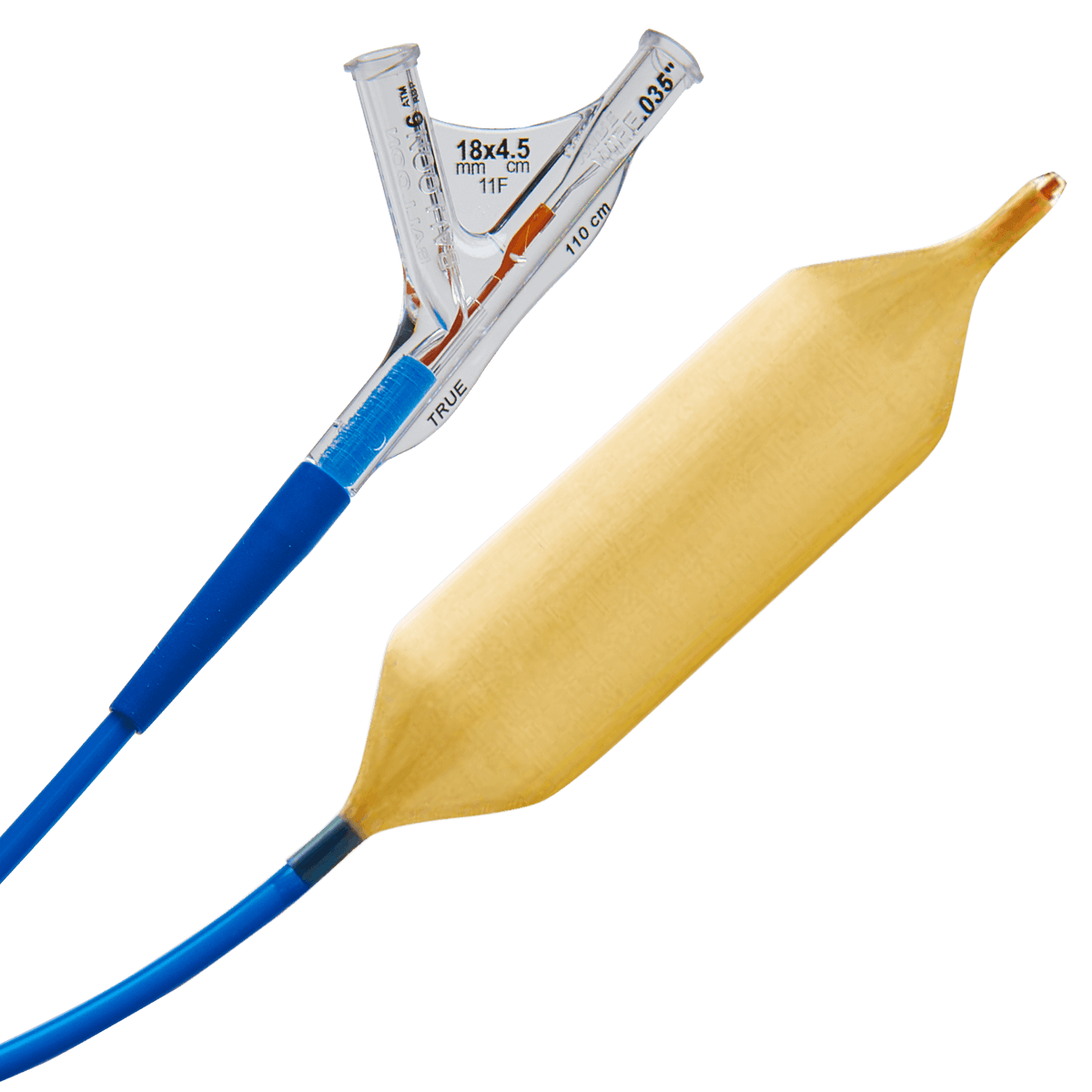
TRUE™ DILATATION
The TRUE™ Dilatation was specifically designed for BAV and TAVI procedures and supports precise, controlled and reliable dilatations.
It is fabricated from KEVLA (mesh-like material) and carbon-coated which makes the balloon highly resistant to ruptures, punctures and tears.
Fiber reinforcements precisely limit the maximum balloon diameter to the labeled size, while allowing flexible conformance to anatomical variations. The TRUE™ Dilatation Balloon redefines the category, offering diameter control within a range of 1.5%.
It inflates and deflates 2 to 3 times faster than competitors’ balloons, considerably minimizing ischemia time.
Cardia, Inc.
These innovative closure devices are inserted via a catheter through the femoral vein into the venous vessel system and offer a safe and reliable method for the minimally invasive treatment of atrial septal defects (ASD) and patent foramen ovale (PFO).
Ultrasept devices are inserted via a catheter in an approximately 30-minute-long procedure by an interventional cardiologist; they can all be deployed without the need for open-heart surgery. This means that the majority of patients who are admitted in the morning may already return home the next day and do not have to go through the lengthy recovery process frequently following open-heart surgery.
Ultrasept PFO
The Ultrasept occluder was developed for the catheter-controlled transvenous closure of patent foramen ovale (PFO).
The occluder consisting of two articulating sails is easily deployed due to its flexible orientation and creates an ultra-low profile within the septum.
When designing the occluder, special focus was put on reducing metal and other foreign materials to a minimum. Patients with an existing nickel allergy, for example, can be treated with the Ultrasept occluder without incurring adverse allergic reactions.
Symedrix GmbH
Symedrix GmbH is a German company headquartered in Oberhaching, Bavaria, specializing in the development and distribution of medical guidewires for minimally invasive procedures.
INNOWI
INNOWI guidewires are mainly used to support and enhance TAVI procedures.
INNOWI as well as INNOWI SX are primarily defined by their special segmentation into three parts with different grades of stiffness. These exactly match the anatomical features and therefore provide ideal support for interventional cardiologists.
INNOWI SX
The wire shaft starts with a very hard segment followed by the yielding segment which lies in the aortic arch and the atraumatic very soft curvature positioned in the ventricle.
The only difference between INNOWI and INNOWI SX is the stiffness of the segment which lies in the aortic arch. Both wires are available in a small and large curvature.
SmartCanula LLC
SmartCanula LLC is an innovative Swiss-based company in the process of solving the technical and practical problems of canulation. The venous smartcanula is the core of the first product line in our portfolio.
smartcanula
Access to the vascular system is a key issue for successful cardio-pulmonary bypass and other types of extracorporeal circulation. The venous smartcanula is a disposable, wall-less canula, for less invasive canulation in conjunction with extracorporeal circulation. Due to the venous smartcanula principle “collapsed canula insertion and expansion in situ”, superior blood flow by smaller access aperture with less trauma results can be achieved.
The venous smartcanula comes together with a mandrel, necessary for insertion. The device is single-use and delivered in sterile condition.
Prytime Medical
Prytime Medical Devices, Inc. is an innovative medical device company that designs, develops and markets minimally invasive solutions for hemorrhage control.
The company’s vision is to revolutionize the care of severely injured patients by enabling trauma teams to gain control of life-threating bleeding as a bridge to definitive repair. The company relies on state-of-the-art technologies and high-quality materials.
The flagship product is the ER-REBOA™ Catheter, a 7 Fr compatible balloon catheter specifically designed for rapid, temporary occlusion of large vessels in emergency and critical care settings during the REBOA procedure (Resuscitative Endovascular Balloon Occlusion of the Aorta).
The underlying intellectual property for the ER-REBOA™ catheter was invented by an experienced military vascular and trauma surgeon.
ER-REBOA™ PLUS
Advancing REBOA™
The ER-REBOA™ PLUS is an advanced, minimally invasive endovascular device that provides rapid and complete aortic occlusion for hemostasis.
The ER-REBOA™ PLUS catheter sets new standards with dual length markers, advanced protection sheath, peel-away sheath and compatibility with guidewires up to .025″.
The unique integrated central aortic pressure monitoring, flexible Nitinol hypotube and patented atraumatic
P-Tip™ ensure precise and reliable aortic occlusion.
The ER-REBOA™ PLUS has been used more than 10,000 times in over 450 hospitals.
AorticLab
AorticLab was founded in 2016 and is an Italian medical device company which is known for its innovative solutions for aortic interventions.
With a consistent focus on research and development, AorticLab is dedicated to advancing the field of cardiovascular care through the introduction of cutting-edge products and technologies.
At the heart of its mission is a commitment to drive innovation for safer and better treatment of aortic valve stenosis. In line with its vision, the AorticLab strives to redefine cardiovascular care through breakthrough technologies. The goal is to increase the confidence of patients and healthcare professionals by providing state-of-the-art solutions.
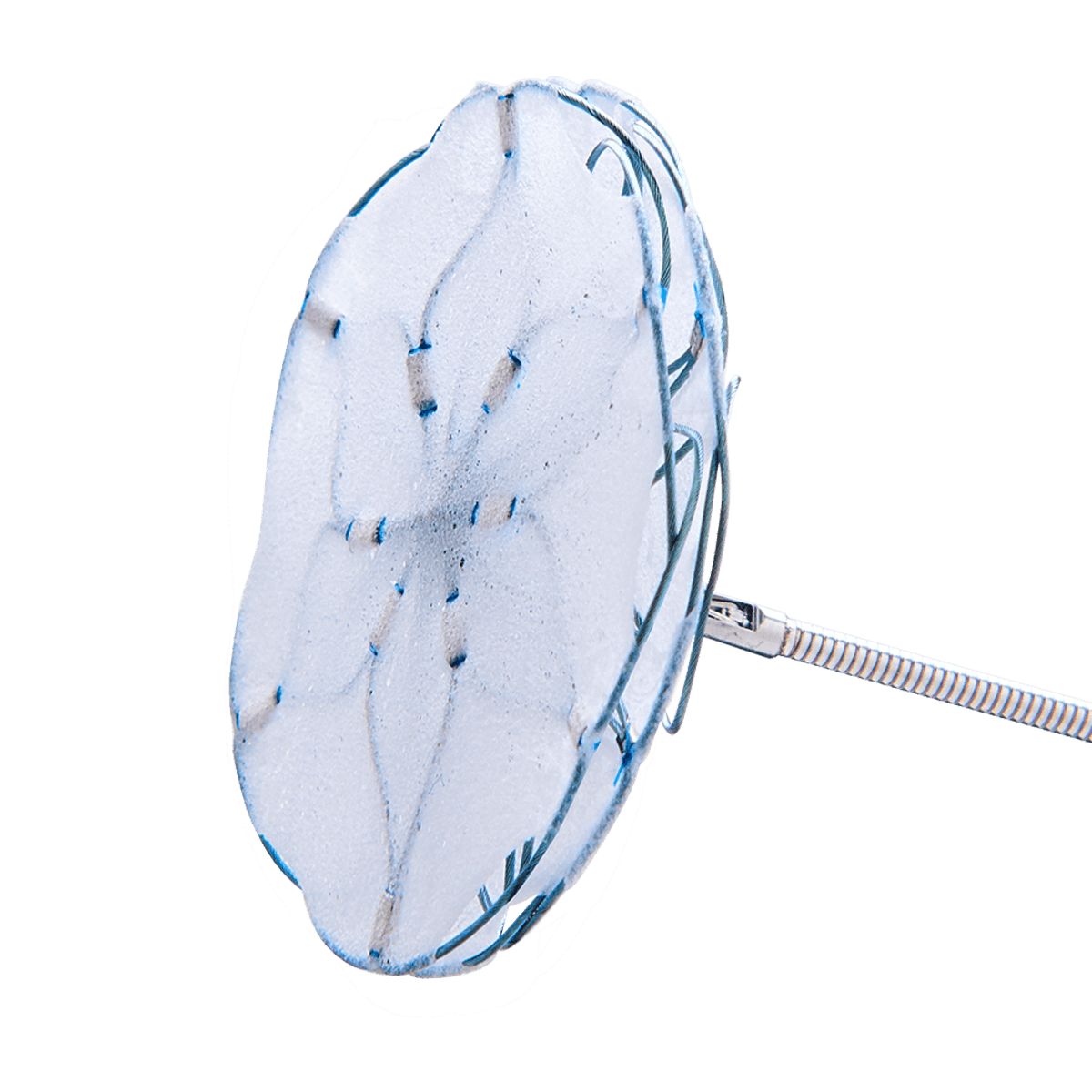
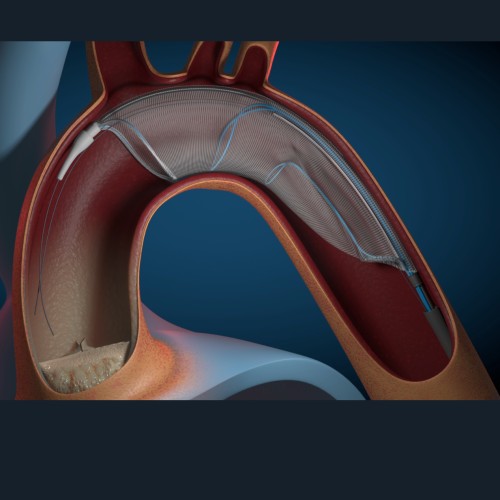
FLOWer
The innovative embolic protection device FLOWer – with a 60 µm mesh – provides secure anchoring in the aortic arch and protects the brain, kidneys and peripheral circulation from the majority of particles released during transcatheter aortic valve implantation (TAVI). As a result, the FLOWer helps reduce the risk of potential complications such as stroke or other adverse effects on vital organs.
With its intuitive design, the device enables fast, easy and safe placement in the aortic arch, a skill that can be easily learned by healthcare professionals.